Product Life Cycle System helps fight COVID-19
Are you working on products which support coronavirus treatment?
Use our Product Life Cycle System platform in your work – for free, for 12 months!
 |
shorten the process of launching new products |
 |
improve R&D works |
 |
simplify the product registration process |
Life Cycle System brings revolution to the pharmaceutical industry!
The system allows for using only one platform during the entire new product development cycle.
Accelerates and optimises pharmaceutical manufacture.
System enables:
 |
Merging data describing activities at all stages of the product life cycle into a single stream of information about product and process. |
 |
Integration with the quality system. |
 |
Continuous improvement of process performance and product quality by identification and control of sources of variation and causes of defective product. |
 |
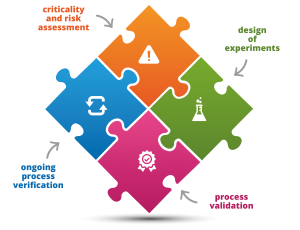
Standarization of technological process development based on the QbD methodology. |
 |
Evaluation of criticality of quality attributes, process parameters and risk of occurrence of OOS results for product attributes based on knowledge gained and experimental data. |
 |
Ongoing real-time verification of the process with identification of atypical trends and OOT/OOS results. |
 |
Visualization of the process monitoring results in real time for persons involved in releasing the product on the market (e.g. Qualified Person). |
 |
Generation of automatic notifications and OOT/OOS alerts for persons involved in the release of the product on the market. |
 |
Monitoring of quality indicators (OOS/OOT, Cpk) and technological indicators (production waste, time of technological operations). |
 |
Integrated portal that enables a holistic view of process performance and product quality throughout the product lifecycle from development to commercial manufacturing. |
For whom?
For research and development departments to implement the QbD approach easily


For quality assurance departments to monitor the process in real time and detect atypical trends
For technological departments to increase effectiveness of medicinal products manufacturing

Product Life Cycle System will definitely make your work easier
StatSoft Polska offers also other solutions for the pharmaceutical industry, not only the Product Life Cycle System. We also supply proven solutions in key areas of pharmaceutical and cosmetic product manufacture.
Reduce your work efforts to the necessary minimum! Learn about our solutions for the pharmaceutical.
If you need help, we are here for you!
info@statsoftpharma.com
+48 601 41 41 51
Our experts have prepared additional materials with which you can broaden your knowledge on the production of medicinal products.
All this should make your work easier!
Feel free to watch our webinars:
Manufacture of medicinal products – from QbD to the verification of the current process. Part 1: Introduction to requirements
Manufacture of medicinal products. Part 2: Analysis of the criticality and risk in the QbD approach
Manufacture of medicinal products. Part 3: Design of experiment (DoE) in the QbD approach
Designing, validation and monitoring of the technological process. Part 1: Assessment of criticality and risk
Read an article by Marek Skowronek, PhD, regarding the manufacture of medicinal products!